When David and I were imagining what this internship would look like, he and I both agreed that we should do an environmental research project that was publishable in a science journal. Ultimately, the publication would benefit people like those in the Peace Corps and missionaries by providing them with information on natural strategies for improving the water situation in their struggling communities.
Besides the obvious goal of improving water for people who desperately need it, other important reasons for publishing a research article are that 1) it looks really good on graduate school applications, 2) it shows the admissions committee that this is, in fact, something I genuinely care about, and 3) that I have worked really hard to use my education to attain the goal of bringing something practical to the water research table. It shows them that they won’t be wasting money and time on someone who just wants another degree, but instead, someone who will use that degree to the greatest potential and to the service of others.
As it came time to start mapping out what this research project would consist of, David and I talked about what we thought was most important. For a while, it seemed like we were on the same page, but in the end, we were not. David had the idea of finding a method using nearby materials to create an inexpensive way of testing the water for various nutrients such as different forms for phosphorus and nitrogen, but I found a cool article talking about how people add common things to the water that attract those nutrients and pull them to the bottom of the water body. Although we had different ideas about whether to tackle the collection method or removal method for overabundant nutrients in water, it is important to note that both methods could be very useful in remote locations with little money. In the end, we started a review of the available published literature over the idea that I had–natural ways to remove nutrients from water–primarily because it would take less time and less resources. David also felt that my heart was headed that way, and that this idea was something that I was interested in, so now we’re running with it.
There are a lot more nutrients dissolved in water than you would think, nutrients that you and I come in contact with every day, nutrients that make it easier for some aquatic life to live and harder for others. In the Cross-Timbers area of Texas, as well as various places across the world, eutrophication is a major issue for rivers, lakes, and ponds. Eutrophication stems, in part, from fertilizers, animal farming productions, wildlife, untreated human waste, and other nutrient-rich materials entering waterways. Nutrients like nitrogen and phosphorus are food for plants. When nutrients are plentiful, it can lead to algae blooms. Algal cells that produce oxygen during the day through photosynthesis, eventually die, settle to the bottom, and are consumed by bacteria that use up oxygen and release carbon dioxide while they breakdown the dead plant material. Dissolved oxygen, the oxygen in water that water animals need to survive, can go very high during the day and very low during the night…so low, in fact, that aquatic animals such as fish can be killed off. The worse the algal bloom, the less oxygen is available. While it is true that some fish are good with little oxygen, all fish must have oxygen to survive. Eutrophication can suffocate aquatic life.
I chose to research phosphorus because, in many cases, phosphorus is the limiting nutrient (meaning that it is normally the scarcest essential nutrient). I also felt that I knew more about phosphorus than nitrogen. When I started to do research, I realized that was a lie. I knew nothing about phosphorus. I stuck with it and read a lot of articles, so I’ll save you the trouble and just tell you the cool stuff that I learned.
In the 1660’s Hennig Brandt, an alchemist in search of the Philosopher’s Stone, discovered the white form of phosphorus while he was boiling his urine (yeah, ew, but phosphorus can now be found in white-yellow, red, violet, black, and pink). When he finally isolated the white phosphorus, he found that it glowed with an eerie green light. Brandt named in phosphorus, the Greek word for “light-bearer”. At the time, Brandt thought that the phosphorus was a good source of light, and a possible alternative to candles (if you could get past the awful smell). Brandt’s maid once picked up some of Brandt’s clothing with a piece of phosphorus on it. He woke up in the middle of the night to a huge fire! The phosphorus had ignited his clothing in his sleep. Unfortunately, pure phosphorus is a highly flammable substance that reacts with oxygen at about 80ºF.
Brandt’s discovery led to the use of phosphorus in everyday modern life and the study of how phosphorus is used within the human body. Phosphates are used in household cleaning supplies such as dishwasher detergent and shampoo, because it helps soften water. The natural flammability of phosphorus led to its use in matches, incendiary bombs, and fireworks. Various forms of phosphorus have been used in pharmaceutical drugs, and the processing and preservation of food. For the human body, as with algae, phosphorus is essential; it actually holds our DNA together and equips our bodies to function at the cellular level.
For plants, lack of phosphorus in the soil can decrease crop yields. Just as there is a cycle of water in the environment, there is a cycle of phosphorus in the environment. Phosphorus is released from rocks by weathering and erosion as a phosphate ion (a group of atoms that has an electrical charge) that is distributed into the soil. Plants take up the inorganic phosphate (has a non-living origin), then they use it for cellular functions similar to human cells. They like the simplest form of phosphates known as orthophosphates, because they are easier to assimilate. When the plants die, the phosphate is returned to the soil in organic form (meaning it was used for life processes). Like I said, phosphorus is commonly the scarcest nutrient for plants. Bacteria, absorption, and the acidity of the soil all have a huge effect on whether the phosphate can be utilized by plants. Most times, plants need more phosphorus than is dissolved in the soil for optimal growth, therefore, farmers add fertilizers to replace the phosphorus once it is taken up by the plants. It sounds as though phosphorus is the most important element in the world, but it is actually extremely toxic in high levels or in its elemental form.
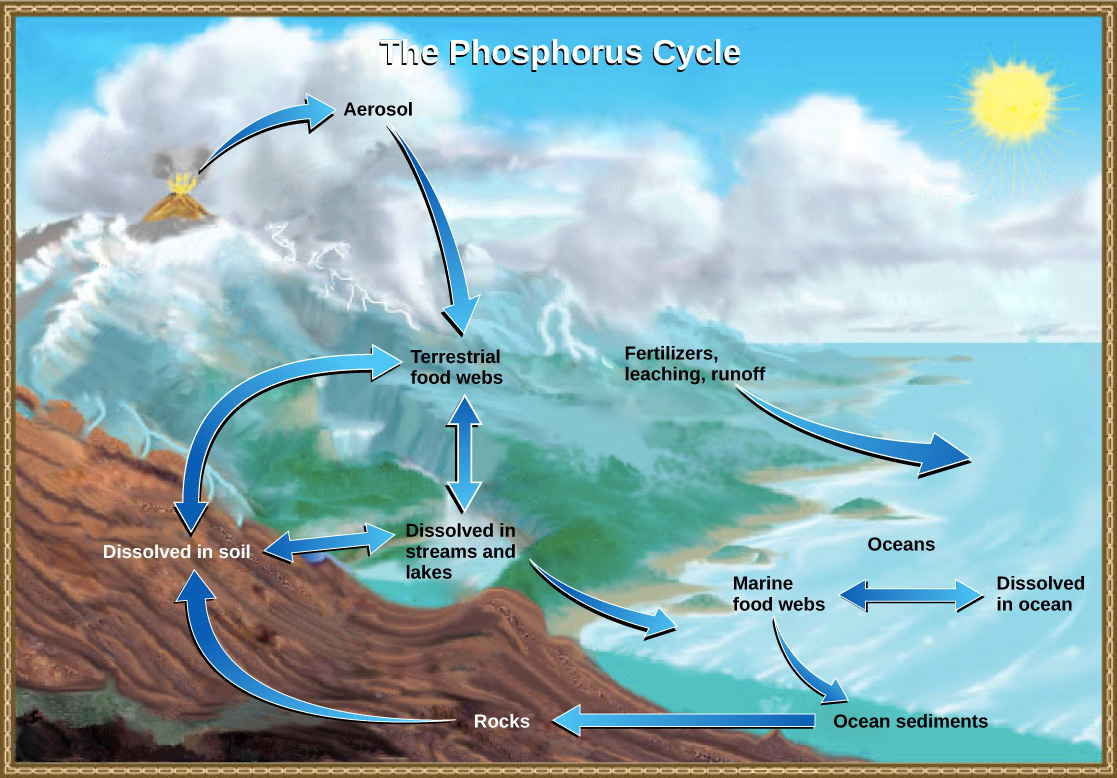
Often times in science, observations are made and explanations are developed using imagination and creativity to help explain theories or ideas. This image of the phosphorus cycle diagram is an explanatory model. These pictures help scientists communicate to the non-scientist public what exactly is going on – sometimes pictures are worth a thousand words.
Although phosphorus occurs naturally in water, too much can really blow things out of proportion. Humans are constantly adding phosphorus to waterways. For example, when farmers, ranchers, or gardeners apply excessive fertilizer, when a flood cleans the floors of a dairy farm, or when a pipe from the local waste water treatment plant has a leak, phosphorus enters a water system in large amounts, a system that is thirsty for that nutrient. When people use cleaning supplies and toss them outside or when pills are flushed down the toilet, chemicals are being added to places they shouldn’t be. These applications can have damaging consequences on miles of fragile stream ecosystems. The water in those ecosystems is necessary for survival, and people have, quite literally, pooped in it.
Thanks for sticking with me through that science talk. Hopefully the next time you purchase shampoo or apply fertilizer to your garden, this blog post will run through your mind. Maybe you’ll see how you are could be contributing to the impairment of water. At the very least, I hope that the next time you see a green carpet of algae floating on a pond, you will have a good idea as to why it is happening and how that ecosystem could be affected by your own use of water and chemicals containing phosphorus.


This is fascinating! And accessible for my non-scientific brain! I had NO idea about phosphorus….